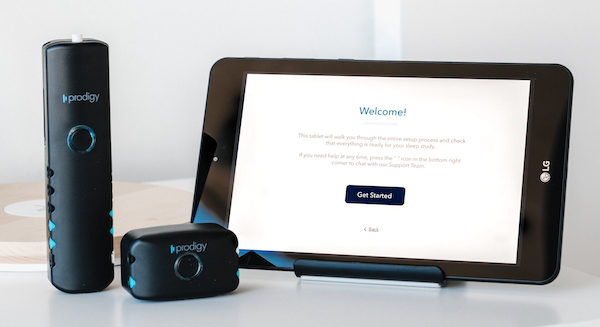
How Might a Type II Sleep System Benefit the Field of Sleep Dentistry? The Cerebra Sleep System
By: Kari Lambing, PhD, Research Scientist at Cerebra
Providing potential alternative treatment options to continuous positive airway pressure (CPAP) for sleep apnea is an important role for sleep dentists.
The standard method of treatment may not always be the ideal solution for a patient as CPAP can come with potential discomfort and some sleep disruption that can lead to low patient adherence (Bachour et al., 2013). The Cerebra Sleep System is an FDA approved Type II sleep testing device that can provide benefits for the diagnosis of sleep apnea, but also provide insight on when alternative solutions may be particularly appropriate for a patient.

Benefits of Type II Systems over Type III Systems (home sleep apnea tests; HSAT)

Type II HSAT
Type III HSAT
Currently, Type III sleep systems are very typical for diagnosis of sleep apnea due to their ease of use for the patient, comfort, and their low cost. These tests measure breathing and oxygen levels, snoring and body position and are completed unattended in the patient’s own home. However, this test misses the mark for recording a key aspect of a sleep disorder- sleep. Previously, the only way to measure sleep was to use the gold standard form of testing, the Type I test.
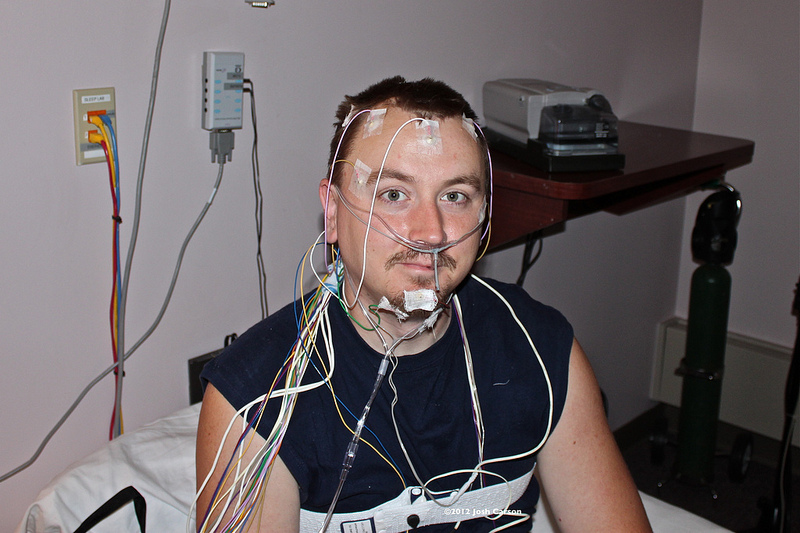
This test occurs in the sleep lab and records more information, such as the electrical activity of the brain (EEG) during the test. However, it is more expensive, more invasive, and less accessible due to geographic barriers. A Type II device shares benefits with both a Type I and a Type III; it is recorded unattended from the comfort of a patient’s own home like a Type III, but it also incorporates the same comprehensive measurements, such as brain activity, as a Type I. Unfortunately, Type II tests are currently underutilized in the field of clinical sleep medicine. However, being able to offer the “best of both worlds” makes Type II systems an emerging key player in the future of sleep medicine.
Inclusion of EEG in home sleep testing has important implications for patient care and diagnosis.
Previous research has highlighted a risk for underdiagnosis of sleep apnea with the use of a Type III system (Kapur et al., 2017). There are two potential mechanisms for this underdiagnosis. The first, is that without the measurement of EEG and arousals, there is not a measurement of respiratory-effort related arousals included in the number of events (Kapur et al., 2017). The second mechanism is that the apnea hypopnea index (AHI) is calculated by dividing the number of respiratory events by the total sleep time.
Without the use of EEG, it forces the use of total recording time in the equation even though some of this time would be before the patient falls asleep and includes any time spent awake during the night (Bianchi & Goparaju, 2017; Kapur et al., 2017). Overall, this results in a smaller AHI, as the number of events is divided over a longer time that includes time where they weren’t even asleep. For some patients with lower sleep efficiency and a greater amount of wake time, this method of calculation could lead to even greater discrepancies in event count and lack of diagnosis (Bianchi & Goparaju, 2017).
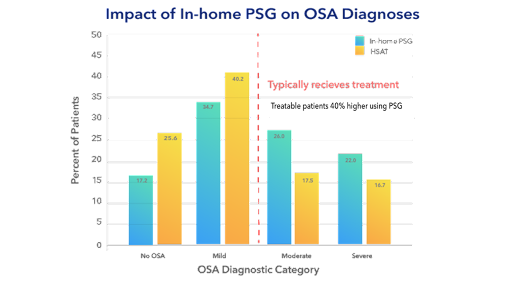
In a currently unpublished study from Cerebra, files were recorded as Type II studies and then had EEG information stripped to simulate Type III HSAT studies. After scoring, it was found that the simulated Type III studies had a lower event count than the Type II studies. 14% of patients in the study were identified as mild sleep apnea with a Type III study but were identified as moderate sleep apnea and could have been treated for sleep apnea when the EEG information was available. This discrepancy potentially led to patients who were left untreated because they weren’t moderate which is typically a requirement to receive treatment for many insurance companies. Overall, the number of treatable patients (having moderate or severe sleep apnea) increased by 40% when using a Type II study over a Type III study (see Figure 1).
Benefits of Cerebra Sleep System
The Cerebra Sleep System has been developed as a Type II device and formulated to bring as much convenience into home sleep testing as possible. The entire setup is accompanied by detailed video and written instructions and electrodes are placed on the forehead which enables it to be fully self-applied by the patient.
This benefit allows the system to be mailed to more remote locations for ultimate patient convenience for testing. Besides the benefits for the patient, there is an online portal for remote study management and as soon as the study is automatically uploaded, it can be accessed by the center’s personnel. If a technical failure or insufficient sleep period necessitates a repeat study, the patient can be simply told to keep it a second night without needing to return the device. The portal can also be used to manage studies, view autoscored studies, and create reports.
ORP and Sleep Improvement
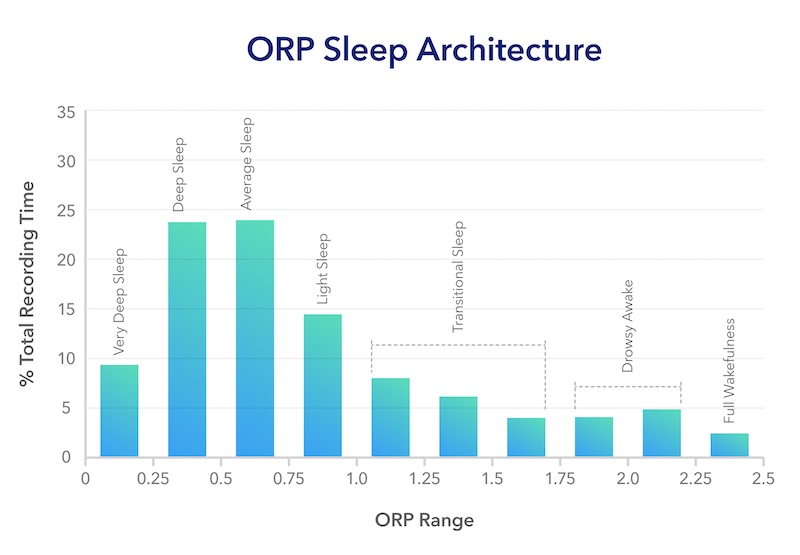
As part of the reports generated by the Cerebra Sleep System, an additional sleep insight is included, Odds Ratio Product (ORP). ORP is a validated measure of sleep depth on a scale from 0 (deep sleep) to 2.5 (full wakefulness). It is calculated based on evaluating the probability of being awake for different patterns of EEG (for more information on how ORP is calculated, see it’s original paper- Younes et al., 2015). It provides a continuous measure of sleep depth measured across the entire night. ORP can provide new insights about the quality of a patient’s sleep to the sleep medicine physicians/dentists beyond those provided by traditional sleep staging (Younes et al., 2015).
Recently, a paper was published on a new way to classify ORP by illustrating the amount of time spent in different depths of ORP over the course of the entire night (Younes et al., 2022a). Different patterns of this distribution were classified into different types based on the amount of time the patient spends in the deepest sleep and full wakefulness.
These distributions provide an easy-to-use visualization of a patient’s sleep, and it is the hope that with more research they may provide insight into the appropriateness of different treatments for different patients. Recent research has already shown that ORP can play a role in predicting adherence to CPAP after 12 months (Younes et al., 2022b), where generally patients who have deeper sleep before diagnosis are less likely to remain adherent to the therapy. This low adherence is likely due to a lower benefit of CPAP for subjective sleep improvement relative to the costs in comfort from the therapy itself. It is perhaps these patients who would have greater likelihood of adhering to a less onerous therapy, such as a dental appliance for treatment of their sleep disorder, as they may not experience many subjective sleep improvements from treatment. As well as guiding therapy, ORP can be used to investigate the response to treatment and how sleep improves.
Conclusion
Overall, the Cerebra Sleep System aims to put the “sleep” back into sleep medicine, by incorporating EEG into home testing that is convenient and accurate for both patient and sleep medicine physicians/dentists.
As part of the Cerebra Sleep System, sleep medicine physicians/dentists have access to the ORP, which ongoing research supports as a valuable measure for measuring sleep depth and quality that shows promise in guiding individualized treatment of sleep disorders.
For more publications about ORP and the Cerebra Sleep System, click the button!
Sources
Bachour, A., Vitikainen, P., Virkkula, P., & Maasilita, P. (2013). CPAP interface: Satisfaction and side effects. Sleep Breath, 17, 667-672. DOI 10.1007/s11325-012-0740-0
Bianchi, M.T., & Goparaju, B. (2017). Potential underestimation of sleep apnea severity by at-home kits: Rescoring in-laboratory polysomnography without sleep staging. Journal of Clinical Sleep Medicine, 13(4), 551-555. DOI: 10.5664/jcsm.6540
Kapur, V.K., Auckley, D.H., Chowdhuri, S., Kuhlmann, D.C., Mehra, R., Ramar, K., & Harrod, C.G. (2017). Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine clinical practice guideline. Journal of Clinical Sleep Medicine, 13(3), 479-504. DOI: 10.5664/jcsm.6506
Younes, M.K., Beaudin, A.E., Raneri, J.K., Gerardy, B.J. & Hanly, P.J. (2022b). Adherence Index: Sleep depth and nocturnal hypoventilation predict long-term adherence with positive airway pressure therapy in severe obstructive sleep apnea. Journal of Clinical Sleep Medicine, 18(8), 1933-1944. DOI: 10.5664/jcsm.10028
Younes, M., Gerardy, B., Pack, A.I., Kuna, S.T., Castro-Diehl, C., & Redline, S. (2022a). Sleep architecture based on sleep depth and propensity: Patterns in different demographics and sleep disorders and association with health outcomes. Sleep, 45(6), 1-15. 10.1093/sleep/zsac059
Younes, M., Ostrowski, M., Soiferman, M., Younes, H., Younes, M., Raneri, J., & Hanly, P. (2015). Odds Ratio Product of sleep EEG as a continuous measure of sleep state. Sleep, 38(4), 641-654. doi: 10.5665/sleep.4588